Strategic metals for a sustainable future
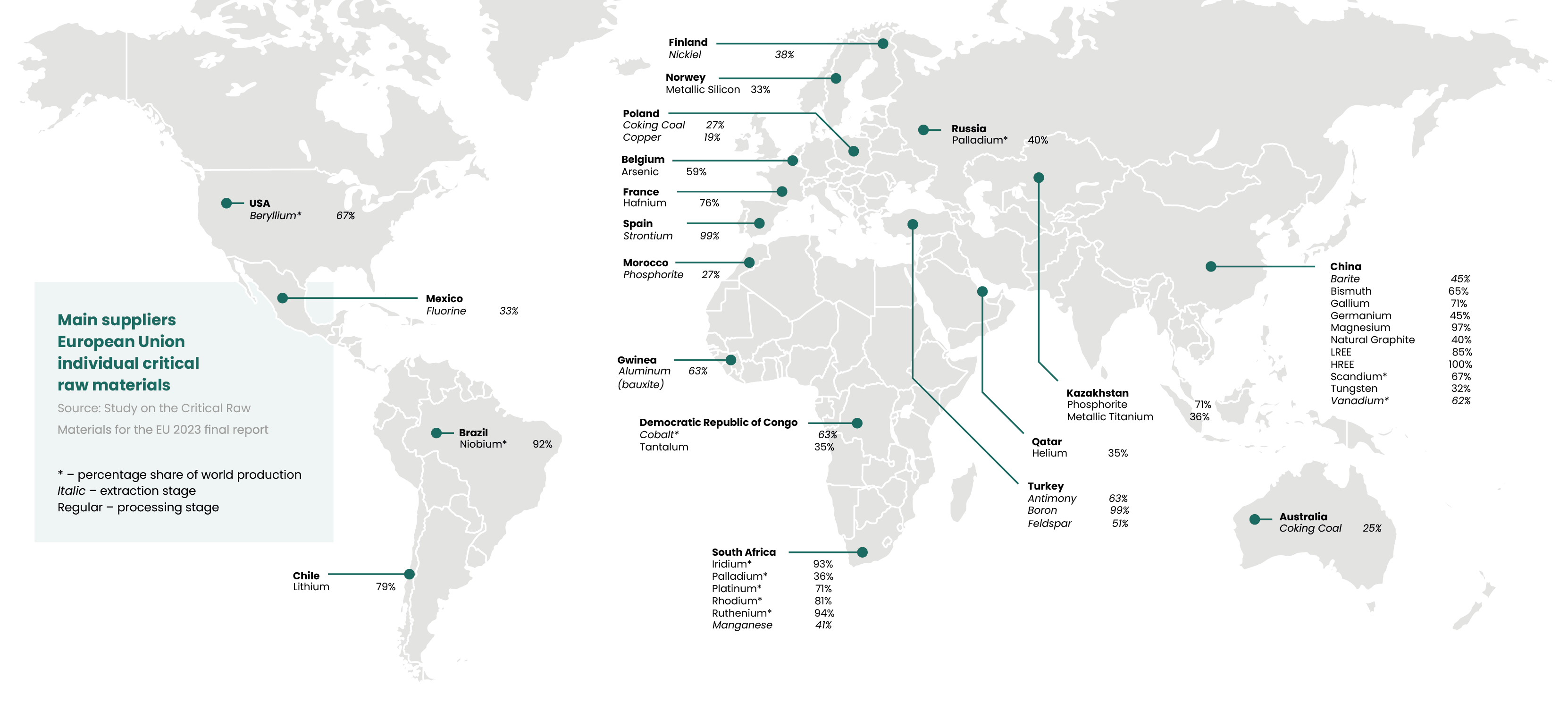
Strategic metals are raw materials that play a key role in industry and are essential for economic and technological development, and their recovery is critical to the future of the economy.
The dynamically developing recycling industry responds to the challenges of growing demand for raw materials, the need to protect the environment, and the need to combat climate change. It plays a key role in promoting a circular economy, reducing dependence on primary raw materials and limiting greenhouse gas emissions through more efficient use of resources in the economy.
Our metals

Lithium
Lithium is a soft, silver-white alkali metal that is the lightest solid element under standard conditions.

Cobalt
Cobalt, like nickel, occurs in the Earth’s crust only in chemically combined form, with the exception of small deposits found in alloys of natural meteoritic iron.

Nickel
Nickel is a silvery-white, shiny metal with a delicate golden hue. It belongs to the transition metals, it is hard and ductile.

Copper
Copper is a soft, malleable and ductile metal with very high thermal and electrical conductivity.

Rhodium
Rhodium is a silver-white, hard, corrosion-resistant and chemically inert transition metal.

Palladium
Palladium is a rare metal, it is shiny and silvery white. It belongs to the platinum group.

Silver
Silver is a soft, white, shiny transition metal that has the highest electrical conductivity, thermal conductivity, and light reflectivity of all metals.

Platinum
Platinum is a dense, malleable and highly non-reactive metal with a silvery-white appearance.

Gold
Gold is one of the least reactive chemical elements. It is often found in a series of solid solutions from native silver, naturally alloyed with other metals such as copper and palladium, or as mineral inclusions.
According to forecasts, by 2040 the demand for copper will increase by 50%
Growing demand in the clean energy sector is increasing the need for key resources
For lithium, a nine-fold increase is estimated by 2040
Demand for nickel, cobalt and rare earth elements will double over the next two decades
Source: International Energy Agency, Global Critical Minerals Outlook 2024